UCB off to a Good Start into 2024 – Progressing on its Growth Path for a Decade Plus
Brussels (Belgium), April 25, 2024 – 07:00 (CEST) – UCB, a global biopharmaceutical company, today shares an update on its progress at its annual shareholder meeting (AGM).
“We are excited to share the progress on our growth path for a decade plus,” said Jean-Christophe Tellier, CEO of UCB. “We had a good start into the year 2024, tracking well towards our full year financial guidance. Our ongoing launches are getting positive feedback from patients and healthcare professionals. The positive prescription trends for BIMZELX® are continuing nicely in all regions where approved and available for patients. Additionally, the first months of 2024 have been marked for BIMZELX ® by five fillings by the U.S. FDA, an approval in the EU, and of course the approval of RYSTIGGO® in the EU”.
Key Growth and Innovation updates
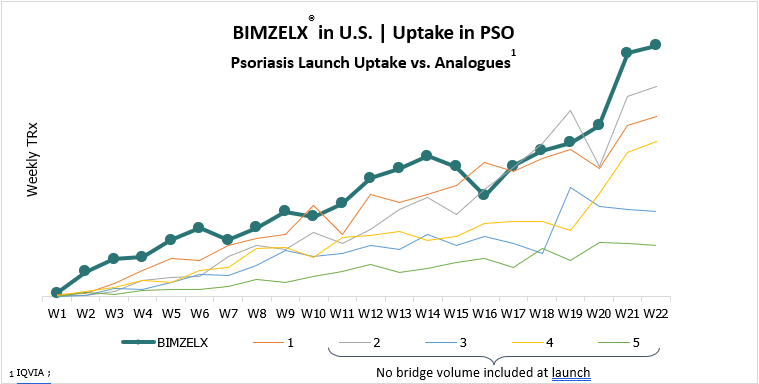
- BIMZELX® (bimekizumab-bkzx) with continued strong uptake in the U.S. BIMZELX®, an IL-17A and IL-17F inhibitor, was approved in the U.S. in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
BIMZELX® is now approved in 42 countries. Five fillings for BIMZELX® (bimekizumab-bkzx) by the U.S. FDA in 2024: applications in psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSpA), ankylosing spondylitis (AS), hidradenitis suppurativa (HS) and for the bimekizumab-bkzx 2mL device presentations. In the U.S., the efficacy and safety of bimekizumab-bkzx in the treatment of PsA, nr-axSpA, AS, HS have not been established and these are investigational indications only.
In March, UCB presented the BIMZELX® four-year psoriasis data at the world’s largest dermatology meeting, showing that the majority of adult patients treated with bimekizumab-bkzx achieved deep and durable clinical response through four years, with a consistent tolerability profile.
“These results, from the largest pool of Phase 3 data, closely follow the U.S. launch, and reinforce our belief that BIMZELX® has the potential to transform the lives of people with moderate to severe plaque psoriasis" said Emmanuel Caeymaex, Executive Vice President, Immunology and U.S. Solutions at UCB.
Earlier this week, UCB received European Commission approval for BIMZELX® as the first IL-17A and IL-17F biologic for moderate to severe hidradenitis suppurativa.
- Approval of RYSTIGGO® (rozanolixizumab) in the EU for the treatment of adults with generalized myasthenia gravis (gMG). (January 2024). March 2024 has been marked by the launches of RYSTIGGO® in the EU and Japan and global launches of ZYLBRYSQ® (zilucoplan) for the treatment of adults with gMG.
“We are very pleased with RYSTIGGO®'s launch uptake in the U.S. Our targeted and customized outreach efforts are effectively connecting us with those in need of our therapies. Meanwhile, following successful launch of ZILBRYSQ® in the first quarter 2024, UCB is proud to continue its support of the gMG community by offering a seamless patient experience with another innovative option,” said Kimberly Moran, Head of U.S. Rare Diseases.
UCB presented 17 abstracts at the 76th American Academy of Neurology (AAN) Annual Meeting, covering new data analyses for UCB’s generalized myasthenia gravis (gMG) treatments, including post hoc and open-label extension results for the approved treatments RYSTIGGO® (rozanolixizumab) and ZILBRYSQ® (zilucoplan).
- In March, the Japanese Ministry of Health, Labour, and Welfare approved FINTEPLA®▼ (fenfluramine) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome (LGS) as an add-on therapy to other anti-epileptic medicines for patients two years of age and older.
- Evolution of UCB’s Executive Committee:
UCB has decided to evolve the structure of its Executive Committee to better align with its commitment to growth and innovation, and to better address the dynamic external landscape of the healthcare industry. The aim is to further capitalize on the solid foundation and legacy established over the years, while incorporating the necessary expertise and leadership to deliver on the next decade of growth and on our innovation.
These are the changes to UCB’s Executive Committee:
Fiona du Monceau will join the Executive Committee to serve as Executive Vice President Patient Evidence. As such she will be responsible for the late-stage clinical development of UCB’s new molecules, brand positioning and strategy, medical affairs and the interaction with key external stakeholders such as government regulators, patient organizations or public officials. Fiona brings extensive expertise of the pharmaceutical industry and has a proven leadership track record.
Emmanuel Caeymaex, currently Executive Vice President and Head of Immunology and U.S., will serve as Chief Commercial Officer. In this new role he will spearhead all commercial activities for the group. Emmanuel’s longstanding tenure at UCB and proven commercial expertise positions him ideally for this new leadership role.
Prof. Dr. Iris Löw-Friedrich, Executive Vice President, Head of Development and UCB’s Chief Medical Officer, will be retiring from UCB. Iris is set to depart later in 2024, ensuring a seamless transition before she embarks on new pursuits.
Dhavalkumar Patel, MD, PhD, Chief Scientific Officer, will be retiring from UCB at the end of June 2024. After a tenure of 7 years, he will pass the baton to a member of his leadership team, Alistair Henry.
Alistair Henry, PhD, currently Head of UK Research, will join the Executive Committee to serve as Chief Scientific Officer. Alistair, who is a biophysicist (PhD, King’s College London) by background, has worked on multiple therapeutic programs through his 26 years at UCB including CIMZIA®, EVENITY® and BIMZELX®. He has championed the use of advanced biophysical methodologies to support discovery and understanding of drugs and the biological systems they interact with. Alistair is renowned for his expertise and unwavering passion for both people and science.
All other members of the Executive Committee will remain in their roles.
“I want to extend my deepest gratitude to Iris Löw-Friedrich, our Executive Vice President and Chief Medical Officer, who has dedicated over 20 years of service to UCB. Iris has been an invaluable member of the UCB Executive team, and during her tenure she has demonstrated exceptional commitment, leadership and expertise which significantly contributed to where we stand today,” said Jean-Christophe Tellier, UCB’s CEO. “I also wish to recognize and thank Dhaval for his vision and passion for science, which has laid a robust foundation for future innovation at UCB. Research and Development is ingrained in UCB’s DNA, and we will further build on the exceptional legacy Iris and Dhaval leave behind. We extend our best wishes to both of them in their future endeavors and on behalf of the UCB Executive Committee, we are pleased to welcome Fiona and Alistair.”
For further information, contact UCB:
Investor Relations
Antje Witte
T +32.2.559.94.14
email antje.witte@ucb.com
Sahar Yazdian
T: +32 2 559 9137
email sahar.yazdian@ucb.com
Corporate Communications
Laurent Schots
T +32.2.559.92.64
email laurent.schots@ucb.com
About UCB
UCB, Brussels, Belgium (www.ucb.com) is a global biopharmaceutical company focused on the discovery and development of innovative medicines and solutions to transform the lives of people living with severe diseases of the immune system or of the central nervous system. With approximately 9,000 people in approximately 40 countries, the company generated revenue of €5.3 billion in 2023. UCB is listed on Euronext Brussels (symbol: UCB). Follow us on Twitter: @UCB_news.
Forward looking statements
This press release may contain forward-looking statements including, without limitation, statements containing the words “believes”, “anticipates”, “expects”, “intends”, “plans”, “seeks”, “estimates”, “may”, “will”, “continue” and similar expressions. These forward-looking statements are based on current plans, estimates and beliefs of management. All statements, other than statements of historical facts, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, arbitration, political, regulatory or clinical results or practices and other such estimates and results. By their nature, such forward-looking statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties and assumptions which might cause the actual results, financial condition, performance or achievements of UCB, or industry results, to differ materially from those that may be expressed or implied by such forward-looking statements contained in this press release. Important factors that could result in such differences include: changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms or within expected timing, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, product liability claims, challenges to patent protection for products or product candidates, competition from other products including biosimilars, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in tax laws or the administration of such laws, and hiring and retention of its employees. There is no guarantee that new product candidates will be discovered or identified in the pipeline, will progress to product approval or that new indications for existing products will be developed and approved. Movement from concept to commercial product is uncertain; preclinical results do not guarantee safety and efficacy of product candidates in humans. So far, the complexity of the human body cannot be reproduced in computer models, cell culture systems or animal models. The length of the timing to complete clinical trials and to get regulatory approval for product marketing has varied in the past and UCB expects similar unpredictability going forward. Products or potential products, which are the subject of partnerships, joint ventures or licensing collaborations may be subject to differences disputes between the partners or may prove to be not as safe, effective or commercially successful as UCB may have believed at the start of such partnership. UCB’s efforts to acquire other products or companies and to integrate the operations of such acquired companies may not be as successful as UCB may have believed at the moment of acquisition. Also, UCB or others could discover safety, side effects or manufacturing problems with its products and/or devices after they are marketed. The discovery of significant problems with a product similar to one of UCB’s products that implicate an entire class of products may have a material adverse effect on sales of the entire class of affected products. Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment, including pricing pressure, political and public scrutiny, customer and prescriber patterns or practices, and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement activities and outcomes. Finally, a breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of UCB’s data and systems.
Given these uncertainties, you should not place undue reliance on any of such forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labelling in any market, or at any particular time, nor can there be any guarantee that such products will be or will continue to be commercially successful in the future.
UCB is providing this information, including forward-looking statements, only as of the date of this press release. UCB expressly disclaims any duty to update any information contained in this press release, either to confirm the actual results or to report or reflect any change in its forward-looking statements with regard thereto or any change in events, conditions or circumstances on which any such statement is based, unless such statement is required pursuant to applicable laws and regulations.
Additionally, information contained in this document shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any offer, solicitation or sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such jurisdiction.
Asset Download
Stay up-to-date on the latest news and information from UCB